Togni reagent II
CAS 887144-94-7, Cat. No EN300-136055
Reagent for trifluoromethylation
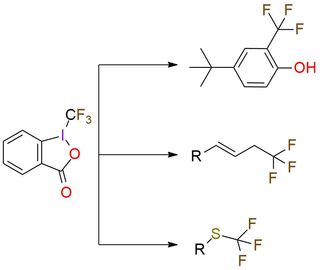
1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one (Togni reagent II) is a highly effective electrophilic trifluoromethylating reagent. It is a colorless crystalline solid, soluble in polar organic solvents. The reagent offers trifluoromethylation of carbon- and sulfur-centered nucleophiles, copper-catalyzed trifluoromethylation of unactivated olefins, phenol derivatives trifluoromethylation, zinc-mediated formation of trifluoromethyl ethers from alcohols1,2. Togni reagent II has a predictable positional selectivity and a high functional group compatibility3. Despite simple procedures and mild reaction conditions, the reagent has explosive properties that are important to remember while working4,5.
Synonyms: 1-(trifluoromethyl)-1,2-benziodoxol-3-(1H)-one; 1-(trifluoromethyl)-1lambda3,2-benziodoxol-3-one; 1-(trifluoromethyl)-1|E3,2-benziodoxol-3-one
Selected publication
1. Copper‐Catalyzed Trifluoromethylation of Unactivated Olefins.Parsons A.; Buchwald S. Angewandte Chemie International Edition 2011, 50 (39), 9120–9123. DOI: 10.1002/anie.201104053
2. Mild Electrophilic Trifluoromethylation of Carbon‐ and Sulfur‐Centered Nucleophiles by a Hypervalent Iodine(III)–CF3 Reagent.Kieltsch I.; Eisenberger P.; Togni A. Angewandte Chemie International Edition 2007, 46 (5), 754–757. DOI: 10.1002/anie.200603497
3. Novel 10‐I‐3 Hypervalent Iodine‐Based Compounds for Electrophilic Trifluoromethylation.Eisenberger P.; Gischig S.; Togni A. Chemistry – A European Journal 2006, 12 (9), 2579–2586. DOI: 10.1002/chem.200501052
4. Notification about the Explosive Properties of Togni’s Reagent II and One of Its Precursors.Fiederling N.; Haller J.; Schramm H. Org Process Res Dev 2013, 17 (3), 318–319. DOI: 10.1021/op400035b
5. 1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one.Stanek K., Koller R., Kieltsch I., Eisenberger P., Togni A. Encyclopedia of Reagents for Organic Synthesis 2009. DOI: 10.1002/047084289X.rn01121
