N-(Benzyloxy)-N-(pivaloyloxy)-4-(trifluoromethyl)-benzamide
CAS 2648454-31-1, Cat. No EN300-33050767
Reagent for N-deleting and deamination
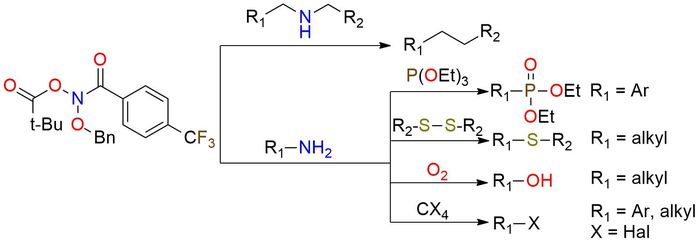
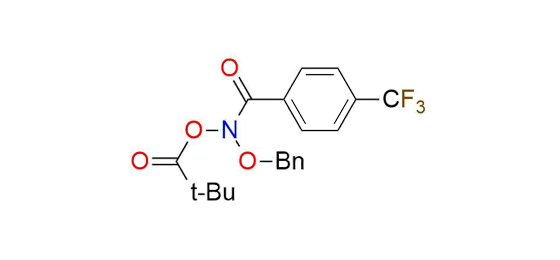
N-(Benzyloxy)-N-(pivaloyloxy)-4-(trifluoromethyl)-benzamide (Levin's reagent) is the unique reagent that enables skeletal editing of molecules through direct nitrogen deletion in secondary amines1. This anomeric amide is a low-temperature-melting yellow solid, stable in soft conditions (0 °C). The reagent reaction with secondary amines follows a simple procedure, resulting in the liberation of nitrogen and forming a C-C bond between the amine substituents. Remarkably, the transformation exhibits an extensive tolerance to various functional groups, allowing for reactions in the presence of hydroxy group, ethers, tertiary amines, thioethers, and protected secondary and primary amines2,3. Levin's reagent enables the single-pot functionalization of primary amines, such as deamination and halogenation2. This capability significantly expands the synthetic possibilities of primary amine transformations.
Synonyms: propanoic acid, 2,2-dimethyl-, (phenylmethoxy)[4-(trifluoromethyl)benzoyl]azanyl ester; (phenylmethoxy)[4-(trifluoromethyl)benzoyl]azanyl 2,2-dimethylpropanoate; Levin's reagent
Selected publication
1. Direct Deaminative Functionalization.Dherange B.; Yuan M.; Kelly C.; Reiher C.; Grosanu C.; Berger K.; Gutierrez O.; Levin M. J. Am. Chem. Soc. 2023, 145 (1), 17–24. DOI: 10.1021/jacs.2c11453
2. Direct Deamination of Primary Amines via Isodiazene Intermediates.Berger K.; Driscoll J.; Yuan M.; Dherange B.; Gutierrez O.; Levin M. J. Am. Chem. Soc. 2021, 143 (42), 17366–17373. DOI: 10.1021/jacs.1c09779
3. Skeletal Editing through Direct Nitrogen Deletion of Secondary Amines.Kennedy S.; Dherange B.; Berger K.; Levin M. Nature 2021, 593 (7858), 223–227. DOI: 10.1038/s41586-021-03448-9
