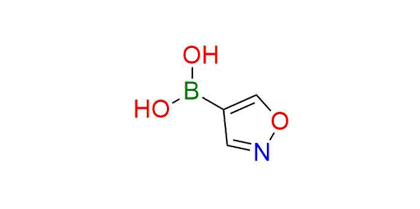
Isoxazol-4-boronic acid
CAS 1008139-25-0, Cat. No EN300-212574
Reagent for DNA-compatible cyanomethylation of (hetero)aryl halides or triflates
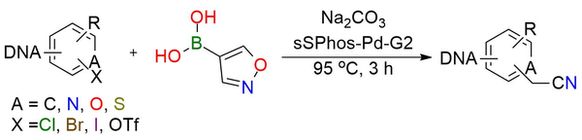
Isoxazol-4-boronic acid is a reagent that offers an easy DNA-compatible, and high-yield cyanomethylation procedure1. It is a solid, bench-stable reagent well soluble in polar solvents. The reaction of cyanomethylation of the heteroaryl halides or triflates proceeds by a tandem process involving palladium-mediated Suzuki−Miyaura coupling and base-promoted isoxazole fragmentation. This process doesn’t damage DNA in a significant way. Heteroaryl halides and aryl triflates containing electron-donating or electron-withdrawing substituent groups can be efficiently converted into the corresponding products under procedure in good to excellent yields. After the reaction, the product can be converted into the corresponding carboxylic acids.
Synonyms: B-4-isoxazolylboronic acid (ACI); (1,2-oxazol-4-yl)boronic acid; isoxazol-4-ylboronic acid; isoxazole-4-boronic acid
Selected publication
1. DNA-Compatible Cyanomethylation of (Hetero)Aryl Halides or Triflates under a Tandem Reaction for DNA-Encoded Library Synthesis.Zhang J.; Wang L.; Ji Q.; Liu F. Org Lett 2023, 25 (37), 6931–6936. DOI: 10.1021/acs.orglett.3c02850
